Pre-Reading
Take a moment to consider the essay prompt for this test:
Does ozone depletion pose a serious threat to life on earth?
As you read, take note of any details you think will help you respond to this prompt in your essay.

Exercise
Open the exercise to begin the activity. Preview the questions, then follow the instructions in the document.
After you are finished, complete the Post-Reading Vocabulary Activity. This will help reinforce the vocabulary relevant to this unit.
Reading 1
The Ozone Layer - A Shield to Earthly Life
Of vital concern to humans and all other life forms on earth is the presence of an ozone layer within the stratosphere. This layer sets in at an altitude of about 15 km (9 mi) and extends upward to about 55 km (35 mi) (Figure 2.9). The ozone layer is a region of concentration of the form of an oxygen molecule known as ozone (03), in which three oxygen atoms are combined instead of the usual two atoms (02). Ozone is produced by the action of solar radiation on ordinary oxygen atoms.
The ozone layer serves as a shield, protecting the troposphere and earth's surface by absorbing most of the ultraviolet radiation found in the sun's rays. If these ultraviolet rays were to reach the earth's surface in full intensity, all exposed bacteria would be destroyed and animal tissues damaged severely. In this protective role, the presence of the ozone layer is an essential factor in the life environment.
A serious threat to the ozone layer is posed by the release of freons, which are synthetic compounds containing carbon, fluorine and chlorine atoms, into the atmosphere. Compounds of this class are also called halocarbons. An alternative name is chlorofluorocarbons, or CFCs. Prior to a ban issued in 1976 in the United States, many aerosol spray cans used in the household were charged with halocarbons. They are also widely used as refrigerants, a practice that continues to contribute to the release of halocarbons.
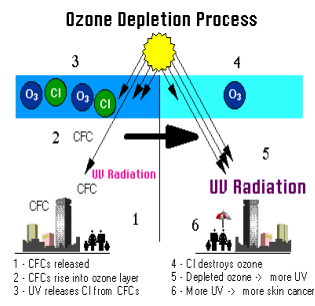
Molecules of halocarbons drift upward through the troposphere and eventually reach the stratosphere. As these compounds absorb ultraviolet radiation (UVR) they are decomposed and chlorine is released. The chlorine, in turn, attacks ozone molecules, converting them in large numbers by chain reaction into ordinary oxygen molecules. In this way the ozone concentration within the stratospheric ozone layer can be reduced and the intensity of UVR reaching the earth's surface can be increased. A marked increase in the incidence of skin cancer in humans is one of the predicted effects. Other possible effects are reduction of crop yields of various plants and the killing of certain forms of aquatic life found in the surface layer of the oceans and in streams and lakes.
As studies of the stratospheric ozone layer accumulated during the 1980s, a substantial decline in the total global ozone began to be indicated. By 1987, following an 8 percent ozone decline in the preceding 8 years, scientists agreed that a serious and persistent global decrease was in progress at a rate far faster than what had been previously predicted.
Compounding the problem of global ozone decrease was the discovery in the mid-1980s of a curious “hole” in the ozone layer over the continent of Antarctica. Here seasonal thinning of the layer occurs during the early spring of the southern hemisphere, reaching a minimum in the month of October. In 1986 a corresponding “hole” was first identified over the Arctic Ocean, occurring annually in February. In both places, the maximum thinning matches the time of lowest surface temperatures. The intensity of the Antarctic hole varies from one year to the next, but a strong general trend to greater seasonal thinning appears to be in progress. Although scientists now confidently attribute the seasonal thinning to presence of CFCs, the mechanisms by which they are made so effective at the poles have been strongly debated.
If the global ozone layer is indeed undergoing a substantial decline in bulk, an increase in the rate of incoming ultraviolet solar radiation (UVR) would be expected and possibly subject to being detected by direct observations. Scientists have estimated that for each 1 percent decrease in global ozone an increase of UVR of 2 percent should result. By 1990, a slight increase in UVR – about 1 percent per year over the preceding 8 years – had been documented at an observing station in the Swiss Alps at an altitude of 3600 m (12,000 ft). In a contrary manner, records at eight stations in the United States over the period 1974-85 clearly show an overall UVR decrease. Many other factors enter in year-to-year changes in recorded UVR, among them variations in average cloud cover, air pollution, and in the content of volcanic dust suspended in the upper atmosphere. For that reason, great caution is needed in linking any observed UVR increase to ozone depletion.
Scientists have warned that if the consumption of CFCs continues at the present rate, they will contribute to an additional atmospheric warming of 2Co (3.6Fo) by the year 2050, over and above the warming predicted for projected increases in carbon dioxide (see Chapter 4). Responding to the global threat of ozone depletion and its anticipated environmental impacts on the biosphere, an international conference (the Vienna Conference), was convened in 1985 by the United Nations Environment Programme (UNEP). Representatives of 43 states brought forth the Vienna Convention of the Protection of the Ozone Layer. Their stated purpose was to promote the exchange of information, research, and monitoring data on human activities that might adversely affect human health and the environment. In 1987, 30 nations endorsed a UNEP plan for cutting global CFC consumption by 50 percent by the year 1999. In 1988, under an agreement known as the Montreal Protocol, the United States joined 31 other nations in approving a similar goal. In 1990, the international agreement was expanded and strengthened to a goal of 50 percent reduction of CFCs by 1995 and 85 percent by 1997. An important part of the program is the substitution for CFCs of the more benign hydrofluorocarbons (HCFCs). Implementing a worldwide phasing out of the use of CFCs will prove difficult to achieve. Compliance will be particularly painful for the developing nations, because the new substitute chemicals are much more costly.
Another and quite different threat to the ozone layer has come from the air transport industry. In 1990, France and Great Britain proposed the building of a fleet of supersonic aircraft using a successor model to the present Concorde. The anticipated fleet of 200 such craft, operating in the stratosphere, is seen by environmentalists as not only contributing to the greenhouse effect by emissions of water vapour, but also destructive to the ozone layer by the emission of oxides of nitrogen.
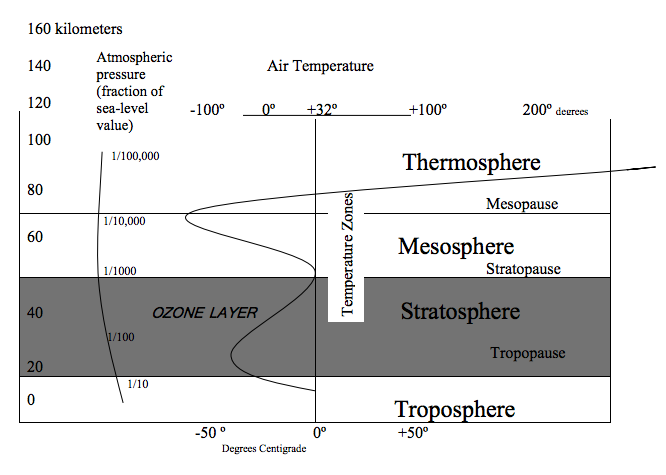
Figure 2.9 - Temperature structure of the atmosphere